

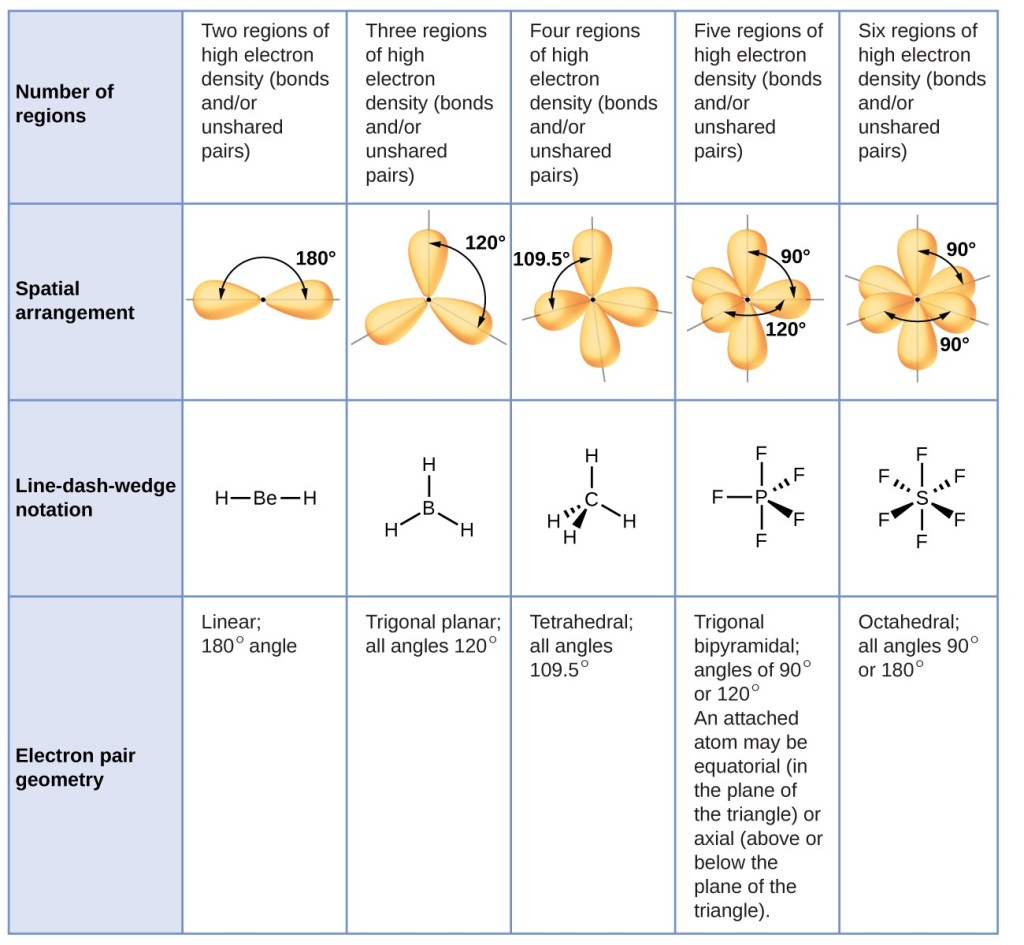
Repulsions are minimized by directing each hydrogen atom and the lone pair to the corners of a tetrahedron.ģ. There are four electron groups around nitrogen, three bonding pairs and one lone pair. The O-S-O bond angle is expected to be less than 120° because of the extra space taken up by the lone pair.Ģ. Thus, with two nuclei and one lone pair the shape is bent, or V shaped, which can be viewed as a trigonal planar arrangement with a missing vertex. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. In SO 2, we have one BP–BP interaction and two LP–BP interactions.Ĥ. Bonding pairs and lone pairs repel each other electrostatically in the order BP–BP < LP–BP < LP–LP. The lone pair occupies more space around the central atom than a bonding pair (even double bonds!). This designation has a total of three electron pairs, two X and one E. With two bonding pairs and one lone pair, the structure is designated as AX 2E. Thus, phosphorus forms three single bonds with Chlorine atoms and one double bond with Oxygen atom.\( \newcommand\)).ģ. As there are double bonds formed between Phosphorus and Oxygen atoms, all the atoms now have a complete octet. In this molecule, phosphorus will share its remaining electrons with Oxygen by forming double bonds. But as it belongs to Group 15 on the Periodic table, it can share all its five valence electrons to attain a stable structure. Oxygen requires two valence electrons to complete its octet.Īfter sharing three valence electrons with chlorine, the Phosphorus atom has eight valence electrons in its outer shell. Thus there are three single bonds formed between Phosphorus and Chlorine atoms.Īfter forming bonds with Chlorine atoms, the Phosphorus atom is now left with two unshared valence electrons as it has shared three out of five electrons with chlorine. Rest all chlorine and oxygen atoms will be placed around this central atom.Ĭhlorine atom needs one valence electron to complete its octet hence it will share one valence electron of phosphorus. Phosphorus atom will be placed in the center as it is the least electronegative out of all the atoms. Here we will look at the Lewis structure of POCl3 to further determine its molecular geometry, bond angle, and shape. The valence electrons that take part in forming bonds are called bonding pairs of electrons, whereas those that do not form bonds are called lone pairs of electrons. The Lewis structure of any molecule helps understand the arrangement of atoms in the molecule, its bond formation, and the valence electrons participating in forming bonds. Total number of valence electrons in POCl3 – Valence electrons of phosphorus + valence electrons of Oxygen + valence electrons of chlorine These valence electrons participate in bond formation.įor finding out the total number of valence electrons for POCl3, we find the valence electrons of all the atoms.Ĭhlorine has seven valence electrons, but as there are three chlorine atoms, we will multiply the number by 3. The electrons that are present in the outer shell are called valence electrons. To determine the Lewis structure of any molecule, we first need to know the total number of valence electrons for the molecule.


 0 kommentar(er)
0 kommentar(er)
